Neural and Myoelectric Implants
Restoring a sense of touch and improving control of prosthetic hands through implantable devices
Overview
We're developing implantable electronic devices to provide advanced control of prosthetic hands and to restore sensations such as touch and finger position.
Press Release:
SALT LAKE CITY, February 9, 2015 – Ripple, a medical device company in Salt Lake City, is developing implantable electronic devices to provide advanced control of prosthetic hands and to restore sensations such as touch and finger position.
As part of the Defense Advanced Research Projects Agency (DARPA)’s Hand Proprioception and Touch Interfaces (HAPTIX) program, Ripple is building a myoelectric implant to detect EMG signals for control of the prosthesis, and an implantable neural stimulator to activate peripheral nerves for restoration of natural sensation.
These devices are planned for use in clinical tests in collaboration with academic research universities.
To accomplish these goals Ripple was chosen by DARPA for a prime HAPTIX contract as a system integrator, as a subcontractor in support of the University of Pittsburgh and Case Western Reserve University, and awarded a Small Business Innovative Research (SBIR) grant.
Total project funding for Ripple in Phase I is $5.9 M with an option for subsequent funding to total $10.1 M. HAPTIX
Advanced prosthetic hands provide exquisite mechanical function, but fail to deliver on the promise of natural control. This is largely due to the absence of subtle sensory signals which provide the feedback necessary for graceful movement. The lack of sensory information from the robotic hand makes the prosthesis seem more like a foreign object than an extension of the body.
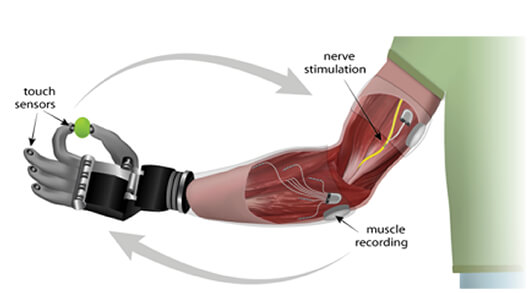
“A hand that cannot feel does not feel like a hand,” explains Dr. Daniel McDonnall, Director of Research at Ripple. Ripple’s myoelectric implant, known as the Myoelectric Implantable Recording Array (MIRA), comprises 32 electrodes that are implanted in an amputee’s forearm to detect EMG signals from residual muscles.
These electrodes connect to a central electronics package implanted subcutaneously which wirelessly sends signals to a receiver built into the prosthetic arm.
The technology was designed for high power coupling efficiency, demonstrated at 50% under nominal conditions. The 32-channel myoelectric implant provides high bandwidth telemetry at 10.2 Mb/s or greater, and is intended to provide simultaneous, multi-degree-of-freedom control of advanced prostheses. The MIRA has been under development for five years with ongoing support from the National Institutes of Health.
A second implantable system under development at Ripple, the Stimulating and Recording Array (SARA), comprises 64 electrodes which selectively stimulate sensory nerve fibers. As with the MIRA, the SARA electrodes connect to a subcutaneously implanted package which wirelessly receives commands and transmits recorded data. HAPTIX seeks to leverage recent advances in understanding the sensory neural code in order to restore naturalistic sensation.
Ripple will coordinate with researchers at the University of Utah, University of Pittsburgh, Case Western Reserve University, the Cleveland Clinic, and Nerves Inc. to conduct clinical trials evaluating performance of these systems in human subjects. Information gathered from these studies will provide support for Ripple’s submission to the Food and Drug Administration for clearance to provide this technology to patients across the nation.
“Our goal is to help people embody their prostheses as an extension of themselves, not only to reduce the challenge of the activities of daily living, but to restore a sense of self to these deserving patients,” says Dr. Daniel Merrill, Chief Clinical Scientist at Ripple.
Blink Prosthesis
Treating facial paralysis through functional electrical stimulation
Overview
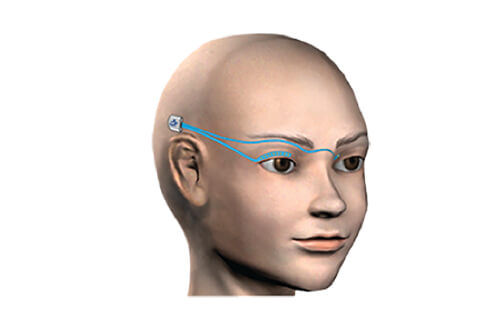
We’re working to restore functional eye blink in patients with unilateral facial paralysis, by creating an implantable blink prosthesis that stimulates paralyzed eyelid muscles to close the eye.
Functional Details
Recording electrodes detect the timing of blink from the healthy eyelid, and then a signal is sent to turn on the stimulator to produce a bilaterally symmetric blink.
Future Outcomes
This innovation will provide a fundamental improvement in the treatment of facial paralysis. These improvements will apply to both the aesthetic and functional use of the paralyzed eyelid by preventing painful dry eye complications and facial disfiguration.
A prototype system has been developed; however, more testing is required before the device will be submitted to the FDA.
Funding
- National Eye Institute
- Concept to Company